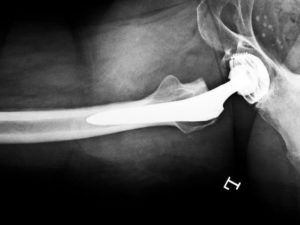
If you or a loved one has injuries or an illness caused by a metal-on-metal hip implant, you deserve compensation. Loncar Lyon Jenkins has settled hundreds of metal-on-metal cases with more pending. We may be able to take on your product liability case in Dallas County and secure the compensation you deserve.
Metal-on-Metal Hip History
In July of 2008, Zimmer Holdings, Inc. issued a voluntary recall of its metal-on-metal Durom Cup due to unusually high numbers of reported device failure. In August of 2010, DePuy Orthopaedics, a division of Johnson & Johnson, recalled its metal-on-metal hip, the ASR, due to unusually high numbers of reported device failure and metallosis.
Metallosis is a buildup of metal debris and infection in the muscle and soft tissue surrounding an implant.
In July of 2012, Stryker Orthopedics voluntarily recalled its hip components, the ABG II and Rejuvinate. While not the classic metal-on-metal design, the devices still have metal components that meet each other that can fail and subject the patient to metallosis.
The Food and Drug Administration (FDA) Gets Involved
By July 9, 2012, the FDA finally decided to conduct hearings on the metal-on-metal hips. The purpose of the hearings was not to assign blame or issue recalls. The purpose of the hearings was to try and come up with a course of action. They wanted to set guidelines for physicians to deal with the massive number of new cases of metallosis and device failure being reported in patients.
Upon acknowledgement that there was a much higher failure rate among the larger sized devices, especially among women, it was advised that doctors should conduct a skin patch test to determine if they were allergic to the device (why this simple test would not be performed prior to the implant of a hip is still unknown).
The Many Questions That Arose
No treatment recommendations were offered. Should a patient with a metal-on-metal hip have routine blood tests for titanium, cobalt, and chromium? When a patient has an abnormal blood metal ion test, what should a doctor do? Does an abnormal blood metal ion test warrant more expensive CTs and MRIs? If the patient is asymptomatic, but the blood test is abnormal, what is the proper course of care?
How abnormal must a blood metal ion test be before surgical intervention is necessary? What if a patient has extremely high levels of cobalt and chromium, but he or she is not healthy enough for another hip surgery? Who is responsible for the costs of the testing, additional procedures, and other expenses related to the hip implant?
The panel concluded more studies were needed, and because of this, the FDA didn’t want to interfere with the doctor-patient relationship. That is it.
Zero Studies Published
Despite nearly 20,000 reported cases of device failure and metallosis and an acknowledgement that the 20,000 reported failures in 2012 was too high, how many studies on these devices have been published since the 2012 FDA hearings?
None.

800-285-HURT (4878)Available 24/7 | 356 Days | se habla español
Legal Options After Suffering Injuries Caused by Metal-on-Metal Hip Implants
Time is running out to file your lawsuit. The manufacturers want you to hope for the best, ignore your pain, and wait for further study while they understand your time to seek compensation for their defective products is running out.
You can do better than crossing your fingers and hoping everything will be okay. It is time to take control of what is happening to you and to protect your rights.
Statute of Limitations for Dallas Metal-on-Metal Hip Implant Lawsuits
If you are hoping to move forward with a metal-on-metal hip implant lawsuit, it is important to connect with an attorney as soon as possible. The statute of limitations for personal injury and medical malpractice lawsuits in Texas is just two years.
Two years may seem like more than enough time to move forward with your case, but the sooner your attorney can get started, the better. Much of the evidence needed to prove liability may only be available for a short period of time.
You also may not be clear when the statute of limitations is going to run out. Will the statute of limitations pass two years from the day your hip implant was installed? Or will the statute of limitations expire two years from the date you learned that your metal-on-metal hip implant was harming you? You can get these answers and more when you connect with a metal-on-metal hip lawyer for legal guidance and support.

800-285-HURT (4878)Available 24/7 | 356 Days | se habla español
Compensation for Victims of Defective Medical Devices
Your life may have been turned upside down by your metal-on-metal hip implant injury or illness. Fortunately, you can seek maximum compensation for your damages. You may be able to file a lawsuit against the manufacturer of these metal-on-metal hips or other liable parties based on the specific details of your case.
In doing so, you could be compensated for every single way your life has been affected by your injuries. To ensure every single loss is considered, your lawyer will separate your damages into categories known as economic and non-economic damages. Here is more information:
Economic and Non-Economic Damages
If you hope to get the most out of your case, your attorney will need to have a clear understanding of all the ways your life has been affected by your injuries or illness. You can be compensated for economic damages (financial losses) and non-economic damages (non-financial losses). Some of the most frequently awarded types of economic and non-economic damages awarded in metal-on-metal hip lawsuits include:
- Physical pain and suffering
- Costs of prescription medications
- Costs of medical equipment
- Future medical care
- Co-pays
- Costs of physical and occupational therapies
- Other medical expenses
- Diminished quality of life
- Unexpected childcare expenses
- Costs of household upkeep and maintenance
- Emotional trauma
- Lost income
- Loss of future potential earnings
- Loss of consortium
Punitive Damages
Since the dangers of metal-on-metal hips are well known at this point, it is possible you could be awarded punitive damages in your lawsuit. If the judge finds the manufacturer’s actions reprehensible or grossly negligent, you could be awarded punitive damages as a means of punishing the defendant for their actions beyond your compensatory damages.
Get Help From a Metal-on-Metal Hip Attorney Today
Loncar Lyon Jenkins will obtain all the records needed to identify the maker of your hip and the model that was implanted. They will analyze your injuries and sue the manufacturer of your hip if necessary.
Contact us before it is too late. We are owed nothing unless we successfully resolve your case, so you have nothing to lose. While we cannot guarantee the outcome of any metal-on-metal hip lawsuits, we can guarantee that if you take no action to protect your rights, nobody will.