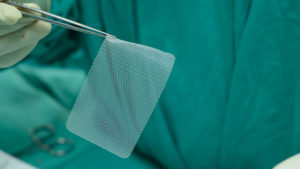
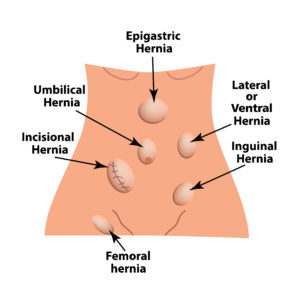
If you have a hernia mesh implant that is bothering you, seek medical attention right away. If your hernia mesh has caused medically diagnosed problems, your time to take legal action may be running out. Many people are unaware that the diagnosis of a problem documented in your medical records may create important legal deadlines that could keep you from receiving compensation. Now is the time to contact our experienced law firm.
A Dallas, TX, hernia mesh lawsuit lawyer with Loncar Lyon Jenkins can help you with a product liability case to recover your medical expenses, lost wages, and other personal injury damages related to hernia mesh complications. We will help you hold hernia mesh manufacturers liable for defective products.
Loncar Lyon Jenkins Is Filing Suits for Individuals Injured by Hernia Mesh
Since 2005, the U.S. Food & Drug Administration (FDA) has recalled over a dozen hernia meshes for varying reasons – mislabeling of packaging documentation, meshes made of counterfeit materials, and design/manufacture defects in the underlying products. In addition, there are hernia meshes not yet recalled that may be defectively designed and causing injuries.
If you have experienced problems with your hernia mesh, particularly with hernia mesh implanted after 2008, contact Loncar Lyon Jenkins immediately about your injuries. Our Dallas product liability lawyers can help you with a hernia mesh claim.

800-285-HURT (4878)Available 24/7 | 356 Days | se habla español
Common Complications of Hernia Mesh
On July 19, 2016, Novus Scientific issued a Class II recall for its TIGR mesh, instructing surgeons to discontinue using the product for the repair of direct inguinal hernias and for the repair of hernias that require permanent support.
Many patients implanted with the defective hernia meshes have undergone additional hernia repair surgeries and have experienced a variety of side effects, including total or partial hernia mesh explant (revision) surgery, hernia recurrences, severe and chronic pain, adhesion, infections, intestinal blockage, and other complications, including:
- Bowel obstruction
- Bowel perforation
- Mesh migration
- Organ damage
- Nerve damage
- Connective tissue damage
If you or someone you love are among those diagnosed with a problem related to your defective mesh, you may qualify for compensation.
Hernia Mesh Lawsuit Damages
Defective medical device manufacturers should not get away with hurting patients who trust their products for help. If you suffered an injury related to defective hernia mesh products, our Dallas hernia mesh lawsuit lawyers can help you seek compensation for:
- Medical bills, including emergency treatment, hernia repair surgery, additional surgery, hospitalizations, and doctors’ visits
- Lost wages, including all income, benefits, and earnings lost due to hernia mesh injuries and your recovery
- Future wage losses, if you have severe injuries and hernia mesh implant complications that cause long-term interference with your ability to make a living
- Physical pain
- Mental and emotional distress
- Inconvenience
- Lost of quality of life
- Wrongful death of a loved one due to fatal hernia mesh side effects

800-285-HURT (4878)Available 24/7 | 356 Days | se habla español
Hernia Mesh and Lax FDA Regulation

There are many types of hernias: inguinal hernia, femoral hernia (very rare), umbilical hernia, incisional hernia (from previous surgeries), epigastric hernia, ventral (abdominal wall) hernia, hiatal hernia. Medical professionals can use mesh in an attempt to repair most types of hernias.
The Ethicon Physiomesh design utilizes polypropylene to manufacture the base layer of the Physiomesh. Polypropylene is the same material that Ethicon utilized to make their transvaginal mesh and bladder slings. Ethicon ( a subsidiary of Johnson & Johnson) has faced thousands of lawsuits over its transvaginal mesh and bladder slings made from polypropylene.
Ethicon added an absorbable film coating to each side of the polypropylene to create the Physiomesh. Ethicon’s Physiomesh received approval for the market in the United States in April 2010. The FDA approved it with a 510(k) application. This allowed the Ethicon Physiomesh to skip rigorous pre-market research and studies.
Ethicon Voluntarily Recalled Hernia Mesh Over Safety Concerns
On May 25, 2016, Ethicon initiated a voluntary, global product recall of the Ethicon Physiomesh due to requests from Ethicon’s own Medical Safety Team.
Unpublished data from two large independent hernia registries (Herniamed German Registry and Danish Hernia Database-DHDB) revealed higher than average recurrence/reoperation rates after laparoscopic ventral hernia repair using the Ethicon Physiomesh when compared to similar products on the market.
Ethicon believes the issue to be a multifactorial issue but, as of yet, has not been able to characterize the exact cause of the product’s defect. The company indicates they will not be returning the Physiomesh to the global market.
Atrium Also Pulled Hernia Mesh From the Market Due to Injuries
Like Ethicon’s Physiomesh, the Atrium C-Qur mesh received approval via the 510(k) application process, forgoing clinical trials.
The FDA cited Atrium in 2012 for ignoring reports of product contamination related to the C-Qur mesh, and production was temporarily halted. A 2013 clinical trial of the Atrium C-Qur V-Patch was cut short due to high infection rates.
On July 19, 2013, Atrium issued a Class II recall for nearly 100,000 units of its C-Qur Mesh — because the coating could peel off and stick inside the package when exposed to heat and moisture. Atrium terminated its recall in March of 2016.
Our Dallas Hernia Mesh Attorneys Seek Justice for Patients
Loncar Lyon Jenkins is a product-liability law firm investigating hernia mesh cases in all 50 states. We are investigating a large number of different hernia meshes for litigation. Hernia mesh lawsuits are being evaluated on a contingency fee basis, meaning you pay nothing unless we secure financial recovery. We will obtain all the medical records needed to evaluate your case, and you DO NOT need to sue your doctor; we pursue product liability cases only.
We can help you join with other hernia mesh plaintiffs in hernia mesh litigation:
Ethicon Hernia Mesh Lawsuits
On April 1, 2016, the first Ethicon Physiomesh lawsuit was filed in the U.S. District Court for the Southern District of Illinois (Matthew Huff v. Ethicon, Inc.). Subsequently, in June of 2017, the United States Judicial Panel on Multidistrict Litigation (“JPML”) consolidated the Physiomesh litigation before the Honorable Richard Story in the United States District Court for the Northern District of Georgia. This is our third time practicing before the Northern District of Georgia in products liability litigation.
Some Ethicon cases have settled for significant compensation. According to Reuters, this includes a $117 million settlement in 2019 involving hernia mesh plaintiffs from 41 states. However, it is not too late to take action against the Johnson & Johson subsidiary for your damages.
Atrium Hernia Mesh Lawsuit
On December 8, 2016, the JPML consolidated twenty-one lawsuits involving the Atrium C-Qur hernia mesh pending in varying federal courts across the country. Lawsuits were consolidated in the District of New Hampshire. It should be noted that this IS NOT class action litigation.
Individual lawsuits will stand on their own, but they will all be litigated in the one federal court in New Hampshire before the Honorable Landya McCafferty. Case-specific discovery is being conducted for the eight trial candidates.
More than 1,500 individual lawsuits have been filed related to the defective Atrium meshes. Some cases have been settled, but many are still pending. It is not too late to become part of a multidistrict litigation (MDL).
Dalvol, Inc./C.R. Bard Hernia Mesh Lawsuits
By August 6, 2018, the JPML consolidated litigation involving specific hernia meshes manufactured and distributed by Dalvol, Inc./C.R. Bard, Inc. in the United States Court for the Southern District of Ohio. The Court is moving quickly. Twelve lawsuits were selected for the initial discovery pool of cases. The third bellwether trial is scheduled for October of 2023.
Individuals implanted with defective meshes governed by this litigation are under strict orders to preserve medical records, medical bills, and pathology samples, including any explanted hernia mesh. If you suffered injuries because of a defective C.R. Bard mesh, you need a hernia mesh attorney immediately.
Take Action for Your Hernia Mesh Injuries Now
Your case is too important to trust to an inexperienced firm or a referral service. Since 1999, Loncar Lyon Jenkins has successfully represented thousands of individuals across America.
You can trust the Dallas hernia mesh lawsuit lawyers at Loncar Lyon Jenkins to provide you with the quality legal representation you deserve. We have been litigating dangerous pharmaceutical and medical device cases since 1999. We can help if you suffered abdominal pain, organ perforation, bowel perforation, needed additional surgeries, or experienced any other hernia mesh complications.
However, do not wait too long to take action, or you could lose your opportunity to recover compensation. Our lawyers will tell you more about statutes of limitations and other deadlines that could apply to your case and help you get started before they expire.
Contact Loncar Lyon Jenkins About Your Hernia Mesh Claim Today
Contact Loncar Lyon Jenkins immediately for a free, confidential case evaluation. Our hernia mesh attorney will obtain all the medical records we need to evaluate your case. You DO NOT need to sue your doctor; we pursue product liability cases only.
Connect with a Dallas hernia mesh lawsuit lawyer near you now. Important legal deadlines may be rapidly approaching. We look forward to successfully representing you.